Case Control
In a Case control study there are two groups of people: one has a health issue (Case group), and this group is “matched” to a Control group without the health issue based on characteristics like age, gender, occupation. In this study type, we can look back in the patient’s histories to look for exposure to risk factors that are common to the Case group, but not the Control group. It was a case-control study that demonstrated a link between carcinoma of the lung and smoking tobacco. These studies estimate the odds between the exposure and the health outcome, however they cannot prove causality. Case control studies might also be referred to as retrospective or case-referent studies.
Stages of a Case control study
This diagram represents taking both the case (disease) and the control (no disease) groups and looking back at their histories to determine their exposure to possible contributing factors. The researchers then determine the likelihood of those factors contributing to the disease.
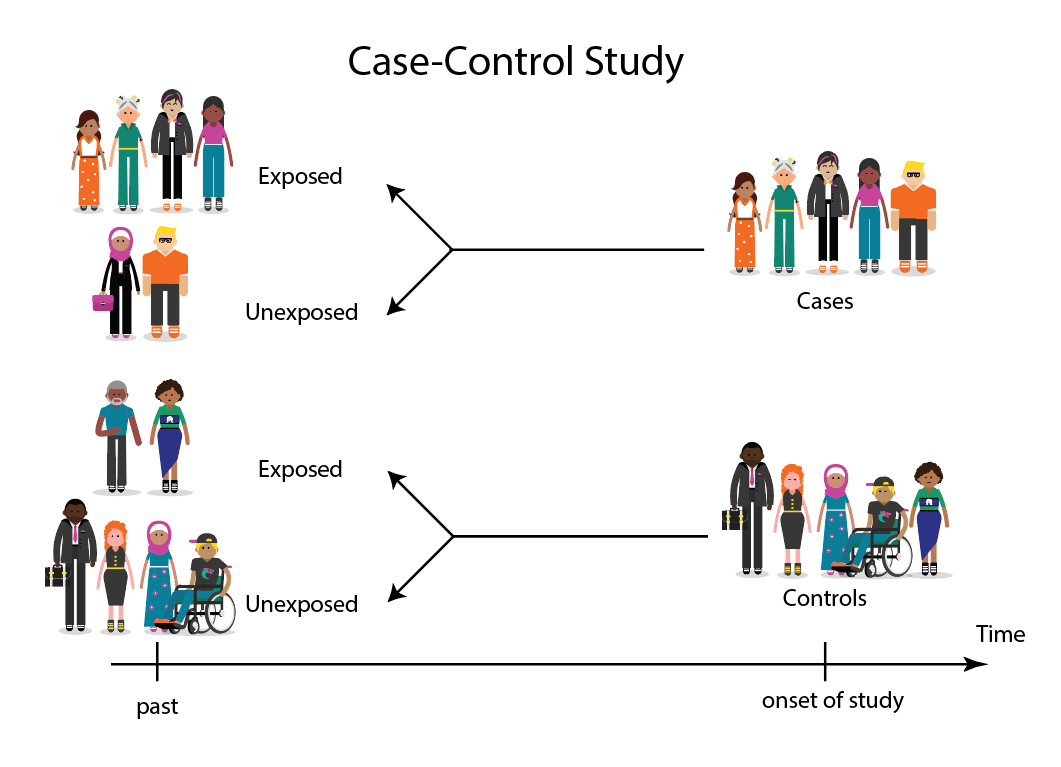
(FOR ACCESSIBILITY: A case control study is likely to show that most, but not all exposed people end up with the health issue, and some unexposed people may also develop the health issue)
Which Clinical Questions does Case control best answer?
Case control studies are best used for Prognosis questions.
For example: Do anticholinergic drugs increase the risk of dementia in later life?
(See BMJ Case control study Anticholinergic drugs and risk of dementia: case-control study)
Considerations when using Case-control study
| Advantages |
|
| Disadvantages |
|
| Opportunities |
|
| Confounding* |
|
* Confounding occurs when the elements of the study design invalidate the result. It is usually unintentional. It is important to avoid confounding, which can happen in a few ways within Case control studies. This explains why it is lower in the hierarchy of evidence, superior only to Case Studies.
What does a strong Case control study look like?
A strong study will have:
- Well-matched controls, similar background without being so similar that they are likely to end up with the same health issue (this can be easier said than done since the risk factors are unknown).
- Detailed medical histories are available, reducing the emphasis on a patient’s unreliable recall of their potential exposures.
What are the pitfalls to look for?
- Poorly matched or over-matched controls.
Poorly matched means that not enough factors are similar between the Case and Control. E.g. age, gender, geography. Over-matched conversely means that so many things match (age, occupation, geography, health habits) that in all likelihood the Control group will also end up with the same health issue! Either of these situations could cause the study to become ineffective. - Selection bias: Selection of Controls is biased. E.g. All Controls are in the hospital, so they’re likely already sick, they’re not a true sample of the wider population.
- Cases include persons showing early symptoms who never ended up having the illness.
Critical appraisal tools
To assist with critically appraising case control studies there are some tools / checklists you can use.
- CASP - Case Control Checklist
- JBI – Critical appraisal checklist for case control studies
- CEBMA – Centre for Evidence Based Management – Critical appraisal questions (focus on leadership and management)
- STROBE - Observational Studies checklists includes Case control
- SIGN - Case control Studies Checklist
Real World Examples
Smoking and carcinoma of the lung; preliminary report
- Doll, R., & Hill, A. B. (1950). Smoking and carcinoma of the lung; preliminary report. British Medical Journal, 2(4682), 739–748. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2038856/
- Key Case control study linking tobacco smoking with lung cancer
- Notes a marked increase in incidence of Lung Cancer disproportionate to population growth.
- 20 London Hospitals contributed current Cases of lung, stomach, colon and rectum cancer via admissions, house-physician and radiotherapy diagnosis, non-cancer Controls were selected at each hospital of the same-sex and within 5 year age group of each.
- 1732 Cases and 743 Controls were interviewed for social class, gender, age, exposure to urban pollution, occupation and smoking habits.
- It was found that continued smoking from a younger age and smoking a greater number of cigarettes correlated with incidence of lung cancer.
Anticholinergic drugs and risk of dementia: case-control study
- Richardson, K., Fox, C., Maidment, I., Steel, N., Loke, Y. K., Arthur, A., . . . Savva, G. M. (2018). Anticholinergic drugs and risk of dementia: case-control study. BMJ, 361, k1315. Retrieved from http://www.bmj.com/content/361/bmj.k1315.abstract.
- A recent study linking the duration and level of exposure to Anticholinergic drugs and subsequent onset of dementia.
- Anticholinergic Cognitive Burden (ACB) was estimated in various drugs, the higher the exposure (measured as the ACB score) the greater likeliness of onset of dementia later in life.
- Antidepressant, urological, and antiparkinson drugs with an ACB score of 3 increased the risk of dementia. Gastrointestinal drugs with an ACB score of 3 were not strongly linked with onset of dementia.
- Tricyclic antidepressants such as Amitriptyline have an ACB score of 3 and are an example of a common area of concern.
Omega-3 deficiency associated with perinatal depression: Case control study
- Rees, A.-M., Austin, M.-P., Owen, C., & Parker, G. (2009). Omega-3 deficiency associated with perinatal depression: Case control study. Psychiatry Research, 166(2), 254-259. Retrieved from http://www.sciencedirect.com/science/article/pii/S0165178107004398.
- During pregnancy women lose Omega-3 polyunsaturated fatty acids to the developing foetus.
- There is a known link between Omgea-3 depletion and depression
- Sixteen depressed and 22 non-depressed women were recruited during their third trimester
- High levels of Omega-3 were associated with significantly lower levels of depression.
- Women with low levels of Omega-3 were six times more likely to be depressed during pregnancy.
Further resources
- Doll, R., & Hill, A. B. (1950). Smoking and carcinoma of the lung; preliminary report. British Medical Journal, 2(4682), 739–748. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2038856/
- Greenhalgh, Trisha. How to Read a Paper: the Basics of Evidence-Based Medicine, John Wiley & Sons, Incorporated, 2014. ProQuest Ebook Central, https://ebookcentral.proquest.com/lib/deakin/detail.action?docID=1642418.
- Himmelfarb Health Sciences Library. (2019). Study Design 101: Case control Study. Retrieved from https://himmelfarb.gwu.edu/tutorials/studydesign101/casecontrols.cfm
- Hoffmann, T., Bennett, S., & Del Mar, C. (2017). Evidence-Based Practice Across the Health Professions (Third edition. ed.): Elsevier.
- Lewallen, S., & Courtright, P. (1998). Epidemiology in practice: case-control studies. Community Eye Health, 11(28), 57. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1706071/
- Pelham, B. W. a., & Blanton, H. (2013). Conducting research in psychology : measuring the weight of smoke /Brett W. Pelham, Hart Blanton (Fourth edition. ed.): Wadsworth Cengage Learning.
- Rees, A.-M., Austin, M.-P., Owen, C., & Parker, G. (2009). Omega-3 deficiency associated with perinatal depression: Case control study. Psychiatry Research, 166(2), 254-259. Retrieved from http://www.sciencedirect.com/science/article/pii/S0165178107004398
- Richardson, K., Fox, C., Maidment, I., Steel, N., Loke, Y. K., Arthur, A., … Savva, G. M. (2018). Anticholinergic drugs and risk of dementia: case-control study. BMJ, 361, k1315. Retrieved from http://www.bmj.com/content/361/bmj.k1315.abstract
- Statistics How To. (2019). Case control Study: Definition, Real Life Examples. Retrieved from https://www.statisticshowto.com/case-control-study/